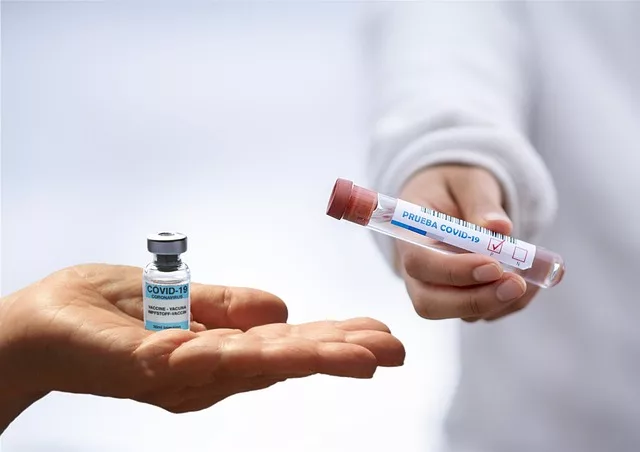
The U.S. Food and Drug Administration (FDA) is currently in discussions with Novavax regarding the potential need for an additional clinical trial as a condition for the full approval of its Covid-19 vaccine, according to a source familiar with internal proceedings.
Novavax, which had expected to receive full FDA approval by April 1, has seen the decision delayed due to the agency’s request for more comprehensive data. The vaccine, which has been authorized for emergency use since 2022, would be the third Covid-19 vaccine to receive full approval in the United States if successful. Unlike the mRNA-based vaccines from Pfizer-BioNTech and Moderna, Novavax uses protein subunit technology, a more traditional vaccine method that some individuals may prefer.
Discussions between the FDA and Novavax are ongoing, and the specifics of any post-approval requirements, including whether another clinical trial must be conducted, have not been finalized. These discussions fall under the framework of Post-Marketing Commitments (PMCs), which are often required by the FDA when it grants full approval for a drug or biologic.
In a recent statement, Silvia Taylor, Executive Vice President and Chief Corporate Affairs and Advocacy Officer at Novavax, confirmed that the company has responded to the FDA’s request regarding PMCs and is awaiting further guidance. “PMCs are common for many approved products. We continue to believe our application meets the criteria for approval and remain committed to working closely with the agency,” Taylor said.
A representative from the U.S. Department of Health and Human Services (HHS), which oversees the FDA, emphasized the agency’s ongoing commitment to safety and science-based regulation. “The FDA’s top priority is ensuring the safety and effectiveness of all products for the American public,” the spokesperson noted.
Meanwhile, the FDA is also reviewing a new class of combination vaccines that protect against both Covid-19 and influenza in a single dose. These innovative dual-protection vaccines could be up for regulatory decisions later this year and are being closely watched by the medical community.
The regulatory process comes during a transitional time at the FDA’s Center for Biologics Evaluation and Research (CBER), the division responsible for vaccine oversight. Recently, Dr. Scott Steele was named acting director following the departure of Dr. Peter Marks. Marks stepped down amid growing public debate around vaccine safety, a topic he addressed directly in his resignation letter, expressing concern over misinformation campaigns.
The shifting leadership and increased scrutiny reflect the larger context of vaccine hesitancy, especially as anti-vaccine rhetoric continues to spread. Public health officials, including those at HHS, continue to reaffirm the importance and safety of vaccines, pushing back against misleading claims, such as those made by public figures falsely alleging vaccines to be dangerous.
As Novavax awaits a final decision, the outcome could have a significant impact not only on the company’s future but also on public confidence in a broader range of vaccine technologies. Full approval would signal a strong endorsement of Novavax’s vaccine and offer more options to Americans seeking Covid-19 protection.
Did you like it? 4.7/5 (22)